Starting Gate at CIRM for $160 Million in Stem Cell and Gene Therapy Research Awards
Call out for "presubmissions" for the cash
California’s stem cell and gene therapy agency will take a deeper dive on Thursday into a new, $160 million award round with different requirements for researchers and new methods of screening applications.
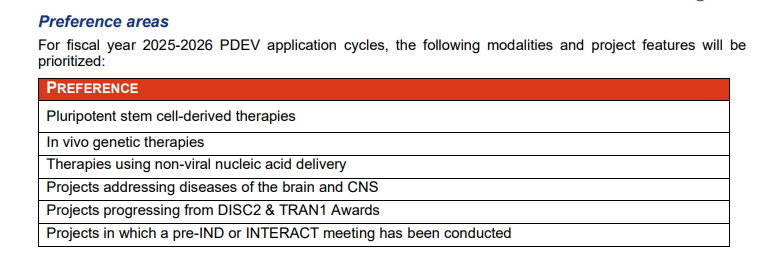
The California Institute for Regenerative Medicine (CIRM) calls it the PDEV round, short for preclinical development. CIRM’s overall goal is “to propel 15-20 therapies targeting diseases affecting Californians to late-stage trials.” The PDEV aim is “to accelerate completion of preclinical development, FDA IND clearance, and clinical trial startup for stem cell-based and genetic therapies.” Patient affordability and accessibility are also considerations.
The program announcement said that the PDEV round was part of “CIRM’s core product development programs that, unlike other funding sources, provide reliable and predictable funding throughout the award period.”
Keep reading with a 7-day free trial
Subscribe to The California Stem Cell Report to keep reading this post and get 7 days of free access to the full post archives.

