Sarepta's Stock Sinks Sharply Following News of Second Death Involving Genetic Therapy
$3.2 million treatment, Duchenne disorder and 'regulatory flexibility' involved
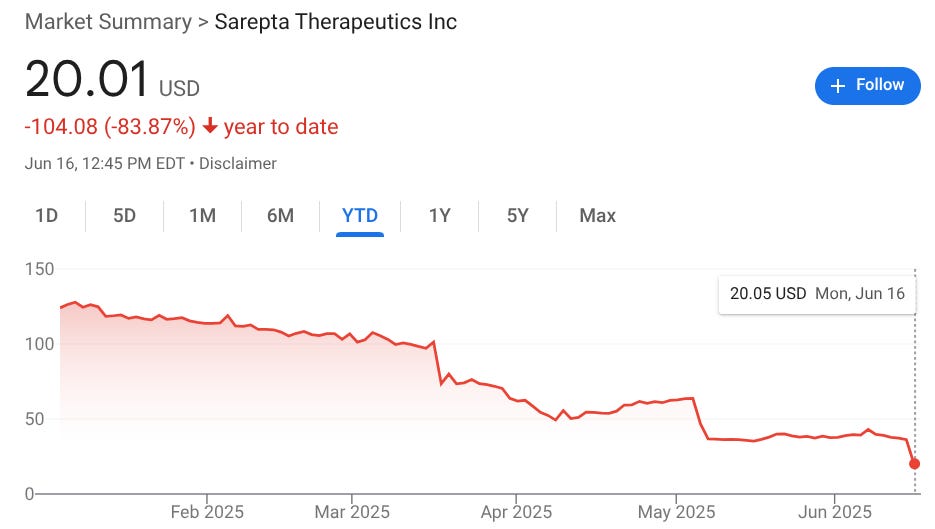
Sarepta Therapeutics’ stock price plummeted nearly 45 percent this morning after the Massachusetts company disclosed a second death involving its multimillion-dollar genetic treatment for a rare disorder called Duchenne muscular dystrophy.
The news also raised concerns about the impact on the gene therapy field more generally, including how the Food and Drug Administration (FDA) would continue its regulatory flexibility. The $3.2 million treatment, known as Elevidys, was approved by Peter Marks, then head of the FDA’s Center for Biologics Evaluation and Research, after he overruled his staff.
Marks’ successor, Vinay Prasad, has been critical of accelerated approvals but recently seemed to be softening his position.
California’s $12 billion gene therapy/stem cell research program appears to have $11 million invested in Duchenne research. The California Stem Cell Report queried the state agency for comment this morning and will carry it if and when it is received. (See our earlier story here.)
Sarepta’s stock price stood at $20.32 shortly after noon EDT, down nearly 45 percent from Friday’s close. Its 52-week high is $173.25. Its 52-week low is $18.32.
Sarepta released the news about its second death at 1 a.m. EDT Sunday.
Keep reading with a 7-day free trial
Subscribe to The California Stem Cell Report to keep reading this post and get 7 days of free access to the full post archives.